Thermal Desorption Spectroscopy (TDS)
TDS is a standard technique used in surface chemistry to determine the binding strength of adsorbates on surfaces or the coverage of these adsorbates (surface particle density). These are important parameters to model the adsorption of a gas-phase species on a surface, as well as for the modeling of surface reactions.
Briefly, for a TDS experiment, the gas is exposed on the surface at low adsorption temperatures. Afterwards, the surface temperature is ramped and simultaneously the intensity of the desorbing particles measured by a mass spectrometer.
The larger the desorption temperature of the adsorbates, the larger the binding strength. The larger the area of the TDS peak, the larger the coverage. A detailed analysis of the obtained curves can yield a number of kinetic parameters, such as the heat of adsorption, the pre-exponential factor, the desorption order, evidence for different adsorption sites on the surface, etc.
![]()
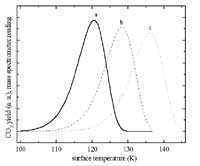
 Most of our papers include also TDS data. Shown here are CO2 TDS curves for a ZnO surface. This system is interesting for the methanol synthesis reaction. The effect of surface defects
Most of our papers include also TDS data. Shown here are CO2 TDS curves for a ZnO surface. This system is interesting for the methanol synthesis reaction. The effect of surface defects
is evident from the high temperature peak.
![]()
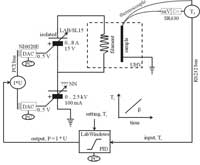
Shown here is a modern computer controlled set up of a TDS experiment as used in our group.
![]()
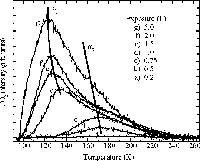
We have a number of home made programs for analyzing these data. Shown here is the result of a computer simulation.